Translate this page into:
Rewiring the upper limb: Motor nerve transfer surgery in the reconstruction of paralysis
2 University Hospital Birmingham, Birmingham, UK
Corresponding Author:
Mohammad Nassimizadeh
The West Midlands Brachial Plexus and Peripheral Nerve Injury Service, Queen Elizabeth Hospital, Midelsohn Way, B152TH
UK
m.nassimizadeh@gmail.com
| How to cite this article: Power D, Nassimizadeh M, Cavallaro D, Jordaan P, Mikalef P. Rewiring the upper limb: Motor nerve transfer surgery in the reconstruction of paralysis. J Musculoskelet Surg Res 2019;3:53-59 |
Abstract
Surgery to rewire a paralysed limb is now possible using motor nerve transfer surgery. The technique has been adapted from brachial plexus surgery and applied to other causes of paralysis with remarkable results, providing function to patients left paralysed from spinal cord injury, inflammatory neuropathy, tumour surgery and degenerative spinal disease. Adapting the techniques to peripheral nerve injury offers improved outcomes compared to anatomical reconstruction of an injured nerve. Nerve transfer surgery involves harvest of an expendable motor branch or redundant motor fascicle from a peripheral nerve and direct, tension-free coaptation to the distal motor branch of a paralysed muscle close to the motor point. Rapid re-innervation of the denervated muscle results in reliable motor recovery. Originally popularised for the reconstruction of nerve root avulsion, the technique has been adapted for use in other peripheral nerve injuries resulting in motor outcomes that are superior to those achieved through grafting of mixed motor-sensory nerve gaps. Nerve transfer surgery may be used to salvage late presenting cases, failed proximal reconstructions or as an adjunct for key motor functions in proximal nerve repairs where the time-distance phenomenon of peripheral nerve regeneration results in poor distal motor recovery, even following acute direct repair. The extension of the technique to other paralysing conditions demonstrates promise.Background
Nerve transfer surgery was described more than a century ago as a method of reconstruction for complex nerve injuries.[1],[2] The technique gained traction in the management of brachial plexus injuries in the last three decades not only as a reconstruction option for nerve root avulsion injuries but also in cases of nerve rupture. The results of targeted nerve transfer for key motor function are more predictable than for nerve grafting where the proximal stump quality is uncertain, re-innervation distances are long and co-contraction of agonists and antagonists results in poor function. Nerve transfers can be used to salvage late-presenting nerve injuries or cases where early nerve reconstruction of a rupture with an autologous graft has failed to achieve functional re-innervation. The time limit for successful nerve transfer is generally accepted to be at between 9 and 12 months following denervation, although many factors contribute to outcomes including the distance of the coaptation from the motor point, the quality of the donor nerve and the extent of the recipient denervation. Studies by Holmes and Young in 1942 and later work by Fu and Gordon in 1995[3] explored the contribution of duration of denervation and delay from neurotomy to motor transfer in animal models and demonstrated that the optimum combination for motor recovery was acute transfer of a freshly transected and uninjured motor nerve to a freshly denervated muscle with a short re-innervation distance.[3],[4],[5] However, denervated muscle can also recover after re-innervation with a freshly cut uninjured donor nerve. This forms the basis for transfer in clinical practice after brachial plexus injuries when there is frequently a delay from injury to reconstruction. Donor and recipient motor axon counts should be matched wherever possible; however, a minimum of 30% of the original motor axon count is needed in the donor nerve for functional restoration with adaptive increases in motor unit size within the recipient muscle. The use of an expendable motor branch as a donor avoids motor-sensory mismatch typical of graft reconstruction of mixed nerve injury. Highly selective fascicle transfer involves the use of intra-neural dissection in a mixed motor-sensory nerve and use of intra-operative stimulation to identify a suitable donor fascicle [Figure - 1]a,[Figure - 1]b,[Figure - 1]c,[Figure - 1]d,[Figure - 1]e,[Figure - 1]f. Although these fascicles will contain both motor and sensory fibres, there is still great recovery potential as a result of the fresh axotomy, the motor axon density and the short re-innervation distance. Careful selection and dissection of the donor fascicle enable preservation of donor function. Between motor branch points, there is distribution of distal motor function throughout the fascicles within a nerve. As the nerve approaches a specific motor branch point, interfascicular branching results in the motor axon supply to the muscle being sector organised into a small fascicle group that will form the motor branch. Sacrifice of one fascicle proximally does not cause complete paralysis of the donor muscle, and the denervated muscle fibres become rapidly adopted by collateral sprouting from remaining nerve fibres at the neuromuscular junction, albeit at the cost of an increase in donor motor unit size.[6],[7],[8]
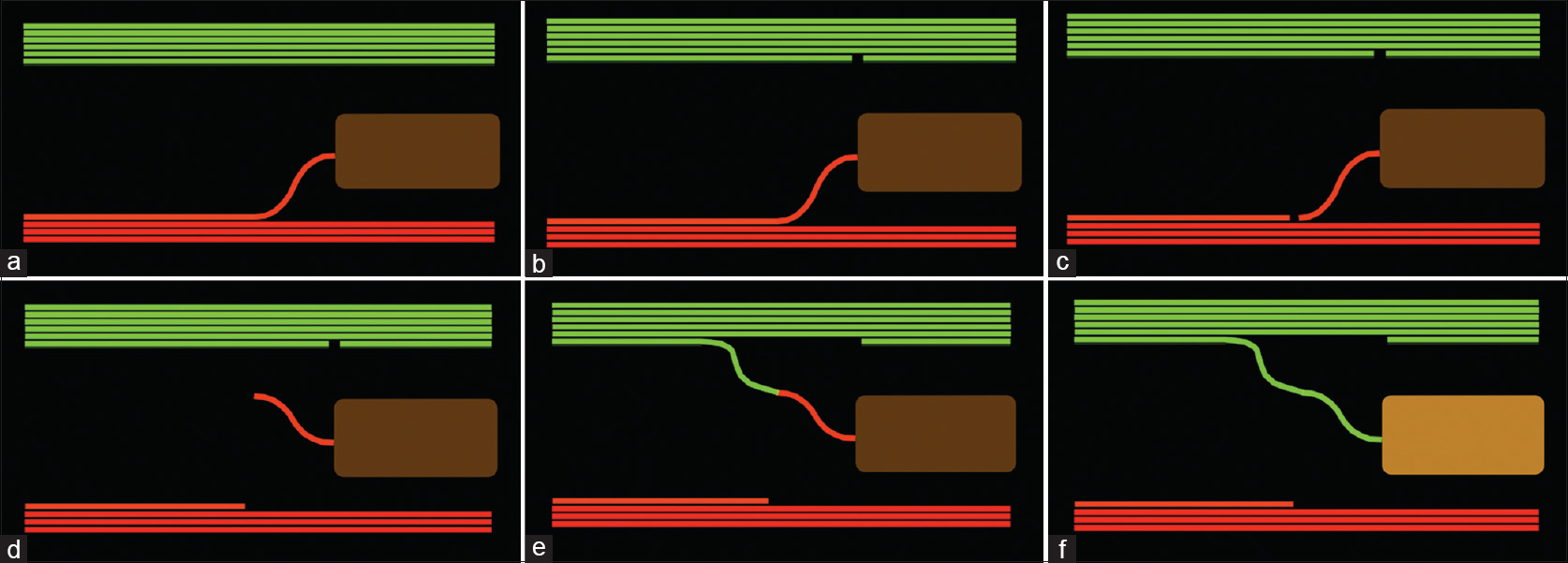 |
| Figure 1: (a) The functioning nerve (green) and the non-functioning nerve (red) with motor branch to a paralysed muscle. (b) A fascicle is selected from the donor nerve and sectioned distally. (c) The non-functioning motor branch is sectioned proximally. (d) The motor branch is mobilised. (e) The donor branch is mobilised and a tension-free coaptation performed. (f) Rapid re-innervation restores function to the paralysed muscle |
Brachial Plexus Root Avulsions
In a C5/C6 nerve root avulsion injury, the functional loss is shoulder abduction, shoulder external rotation and elbow flexion. The spinal accessory nerve may be transferred to the supra-scapular nerve for restoration of supraspinatus and infraspinatus. Critical review of clinical results demonstrated some failures and generally poor external rotation. The posterior approach using the medial spinal accessory nerve branch transferred to the supra-scapular nerve distal to the released supra-scapular ligament allows closer coaptation to the muscles and prevents re-innervation of the nerve proximal to a distal neuroma that may result at the notch following the tractional injury to the shoulder girdle. Witoonchart et al. recognised that shoulder abduction was sometimes inadequate following the spinal accessory nerve transfer as a single transfer for the shoulder and they reported a second transfer to the deltoid using the long head of triceps branch through a posterior approach.[9] Further refinements of this approach have been suggested by Colbert and Mackinnon including transfer of the medial triceps branch to both anterior and posterior divisions of the axillary nerve to allow re-innervation of teres minor as well as deltoid.[10],[11],[12] Elbow flexion restoration can be achieved using a transfer of an ulnar nerve fascicle to the motor branch to biceps,[13],[14],[15] with the option of a second transfer from the median nerve to the nerve to brachialis.[16],[17],[18] There is a greater proportion of Medical Research Coucil (MRC) grade 4 outcomes with a double nerve transfer for elbow flexion than a single transfer; however, the added functional benefit of a second nerve transfer for elbow flexion is not as great as that seen in restoration of shoulder abduction and external rotation.[19],[20] In lower root avulsions C8/T1, the options for nerve transfer are limited by the longer re-innervation distances to the more distally located denervated extrinsic flexors and intrinsic muscles. The nerve to brachialis may be transferred to the anterior interosseous nerve (AIN) for finger and thumb flexion. This transfer must be performed soon after injury because the re-innervation distance is typically 8 inch. The nerve branches to supinator may be transferred to the rest of the posterior interosseous nerve (PIN) for finger and thumb extension.[21],[22],[23] Currently, intrinsic restoration through nerve transfer results in poor functional results.
Failed Primary Nerve Surgery
The reliable results of nerve transfer in nerve root avulsion injuries have resulted in many surgeons using nerve transfer as a primary reconstruction option even in cases of nerve root or trunk rupture. However, in many cases of adult traumatic brachial plexus injury, there is a pan plexus pattern of involvement at presentation, and although there may be physical continuity of the lower plexus, there is inevitably some degenerative axonopathy typical of the true mixed nerve injury and the option of highly selective fascicle transfer from branches of the lower plexus for restoration of upper trunk function is uncertain. In these cases, autologous graft reconstruction of the nerve gap can be performed early. Later interventions targeting key distal muscle groups with nerves transfers can be used for poorly regenerating nerves, should a suitable donor become available. An example would be a hybrid reconstruction of an upper trunk rupture with spinal accessory transfer to the supra-scapular nerve early plus autologous nerve grafting of the upper trunk. If either deltoid or biceps recovery is poor, then targeted distal nerve transfers from the triceps,[10] and the ulnar nerve[13] can be undertaken successfully between 6 and 9 months if the lower plexus recovery is satisfactory. This approach can be used in isolated peripheral nerve injury where a proximal primary repair has resulted in poor recovery due to the formation of a neuroma at the repair site. A targeted distal nerve transfer can be performed for a key function.
Case discussion
A 26-year-old male soldier sustained a high-velocity gunshot wound to the lateral aspect of the upper arm with transection of the radial nerve. After debridement, the nerve gap was reconstructed using cables of autologous sensory nerve. There was a poor progression of the Tinel's sign with a persistent strong Tinel at the proximal neurorrhaphy site. There was no recovery of brachioradialis at 6 months and electromyography demonstrated no evidence of re-innervation. The options for reconstruction included re-exploration and debridement with repeat autologous nerve grafting, nerve allograft reconstruction, tendon transfers or distal nerve transfers. The proximal nature of the injury, the uncertain quality of the proximal nerve stump and the duration of denervation are poor prognostic indicators for repeat grafting, and the patient opted for nerve transfer surgery. The median nerve was exposed in the proximal forearm and the branches to flexor carpi radialis (FCR), palmaris longus (PL) and flexor digitorum superficialis (FDS) identified and confirmed using nerve stimulation. Using rubber slings, the nerve branches were neurolysed proximally to gain length for transfer. The radial nerve was exposed in the interval between brachioradialis and brachialis. The branch to extensor carpi radialis brevis (ECRB) and the PIN were tagged and neurolysed. The branches to supinator from the PIN were excluded. The FCR and PL fascicles were transferred to the PIN and the FDS branch to the ECRB. Both coaptations were sutured using the operating microscope and augmented with fibrin glue.[24],[25] Muscle tenderness at 3 months indicated re-innervation with small fibres, voluntary contraction at 6 months with strengthening and useful motor function at 12 months following the nerve transfer salvage surgery.[26]
Late Presentation of Peripheral Nerve Injury
When a late diagnosis is made of a peripheral nerve injury, re-innervation of key motor targets may be best achieved through targeted distal nerve transfer due to the time-distance phenomenon precluding useful motor recovery if the denervated muscle cannot be reinnervated by 12 months. Proximal surgery with excision of neuroma and autologous grafting may still be considered for pain management, restoration of motor function in proximal muscles or sensory recovery.
Case discussion
A 24-year-old man was referred to the nerve injury clinic 7 months after a shoulder dislocation with axillary nerve palsy. The first examination did not diagnose the axillary injury, and a treating physiotherapist managed the patient expectant of a full recovery from a neurapraxic injury. Reassessment in the shoulder clinic at 4 months demonstrated no recovery, and the patient was referred for neurophysiology studies including electromyography. The studies confirmed a complete axillary lesion with no demonstrable continuity and no evidence of re-innervation. The patient was referred to the nerve clinic for further evaluation. Anterior exploration and nerve grafting were discussed; however, the late presentation means that useful recovery could not be guaranteed if the rupture was proximal from the posterior cord. A distal nerve transfer from the medial triceps branch to the axillary nerve through a posterior approach was performed at 8 months following injury.[10] Six months following nerve transfer, there was useful motor recovery in the deltoid which strengthened to MRC grade 4 over 18 months.
Distal Augmentation of a Proximal Reconstruction
Following nerve grafting of the upper trunk, biceps may recover at 9–12 months from surgery. The more distally placed brachialis may not achieve useful re-innervation due to the additional distance required for axonal re-growth. In such cases, a targeted fascicle transfer from the ulnar nerve to the motor branch to brachialis[16] may result in improved elbow flexion function using this hybrid approach. This approach is usually employed in cases where the option for nerve transfer is not available from the outset due to a pan plexus presentation. In such cases, there may be a rupture of the upper trunk requiring autograft reconstruction, and there may be a mixed nerve injury or prolonged conduction block of the inferior plexus that following rapid recovery in the first 3–6 months becomes suitable for fascicle harvest for augmentation distal nerve transfer.
Case discussion
A 60-year-old male motorcyclist was involved in a road traffic collision sustaining multiple injuries requiring a prolonged critical care stay. After transfer to the ward at 4 months, it was noted that there was no function within the right biceps or brachialis. There was a Tinel's sign in the infra-clavicular plexus radiating to the lateral cutaneous nerve of the forearm territory. The medial cord demonstrated poor function indicative of a mixed nerve injury. Flexor carpi ulnaris (FCU) was MRC grade 3 on examination. Exploration of the infra-clavicular plexus revealed a rupture of the musculocutaneous nerve at the coracobrachialis. The gap was grafted using autologous sural nerve cables. Recovery was monitored, but the rate of Tinel's progression was slow, and following spontaneous recovery of the FCU to MRC grade 4, the option of a distal nerve transfer was discussed. At operation, the nerve to biceps was exposed and stimulated demonstrating some recovery through the graft. There was no contraction of the brachialis on stimulation. A distal fascicle transfer to the brachialis from the ulnar nerve was performed at 10 months.
Adjunct to Proximal Nerve Repair
Following acute complete proximal nerve transection, the potential for distal recovery is dependent on early anatomical repair and the distance for motor re-innervation to distal targets. Typically, a repaired nerve regenerates at 1 mm per day, and if motor axons do not reach the denervated target by 9–12 months from injury, then permanent paralysis results. In cases of high ulnar nerve transection at or above the elbow, an acute repair can be augmented by transfer of the distal AIN from pronator quadratus to the motor fascicle of the ulnar nerve in the distal forearm.[27] This should only be performed after confirming that the patient has full denervation of the ulnar innervated intrinsic without a Martin–Gruber anastomosis. The motor fascicle lies sandwiched between the smaller dorsal sensory branch and the larger main sensory fascicle in the distal forearm approximately 7 cm proximal to the pisiform. The AIN is a good size match for half of the motor fascicle, which can be readily split into component fascicles through intramural dissection. Each half of the motor fascicle contains innervation to all intrinsic, and the motor fascicle is not sector organised at this level.[28] Transfer of the AIN to one half of the motor fascicle as an end-to-end transfer allows re-innervation of the intrinsic within 6 months with the possibility of a second wave of re-innervation from the proximal regenerating axon front at a later date. The early re-innervation prevents irreversible endplate degeneration and extends the window for successful re-innervation despite the proximal site of the primary nerve repair.
Case discussion
A 35-year-old man was assaulted and pushed through a window sustaining a complete transection of the ulnar nerve and the triceps tendon above the elbow. The triceps was repaired and the ulnar nerve sutured with microscope assisted direct neurorrhaphy. A distal hemi-AIN to motor fascicle ulnar nerve transfer was performed. During the recovery, the re-innervation of the flexor digitorum profundus did not result in claw posture of the hand and no anti-claw splint was required. There was some electromyographic recovery of the abductor digiti minimi at 6 months post-injury and the patient went on to achieve useful intrinsic recovery after 2 years.
Reconstruction of Peripheral Compression Neuropathy
Compressive neuropathies are common, but complete distal motor loss is rare. The sensory symptoms usually result in early referral and intervention. Motor weakness in the thenar muscles from carpal tunnel compression may improve after surgical decompression, but complete wasting and muscle atrophy are usually permanent. The proximal nature of cubital tunnel compression and the distal ulnar innervated intrinsic mean that distal recovery is uncommon when there is severe motor weakness or wasting at presentation. Surgery for the compression site may prevent further loss of sensory function and reduce pain; however, the lack of intrinsic function results in poor hand function. The window for successful re-innervation using a distal nerve transfer is unclear due to the duration of compression, the progressive nature of compressive neuropathy motor axon loss and the possibility of adoption at the neuromuscular junction with a few axons with large motor units maintaining muscle fibres in a condition suitable for re-innervation although at a non-functioning level. The option for a salvage distal nerve transfer from the AIN to the motor fascicle of the ulnar nerve in the distal forearm is one that can be contemplated although there are no published outcome data to support this approach currently.[29]
Case discussion
A 70-year-old man presented with severe cubital tunnel syndrome with wasting of the ulnar innervated intrinsic muscles. A cubital tunnel release and medial epicondylectomy was performed with a distal AIN to motor fascicle ulnar nerve transfer above the wrist. The intrinsics remained wasted at 12 months; however, subjectively, the patient reported improved control or the ulnar digits and there was some evidence of recovery of activity in the abductor digiti minimi.
Reconstruction After Spinal Nerve Root Compression
Spinal nerve roots may be compressed as part of degenerative spondylosis with direct compression from far lateral disc prolapse and loss of disc height with osteophyte encroachment causing a foraminal stenosis. Onset of compression may be acute or slow and insidious. Complete loss of motor function in the root causes motor weakness or paralysis if there is single root innervation for a given muscle. Nerve transfers in the peripheral may be used for reconstruction as long as the donor is not compromised. The window for successful re-innervation is not known and probably depends on the duration of paralysis, the rate of axon death, residual axon density in the compromised nerve and the presence of adoption within the denervated muscle.
Case discussion
A 54-year-old man presented with deteriorated function after a C5 foraminotomy for degenerative root compression due to mid-cervical spondylosis. There was no functional recovery to deltoid and electromyography demonstrated complete denervation with no evidence of re-innervation by 9 months. A nerve transfer from the long head of triceps to the anterior division of the axillary nerve through a posterior approach restored MRC grade 4 function in the deltoid at 12 months post-surgery.
Reconstruction After Inflammatory Neuropathy
There are a group of inflammatory neuropathies of varying and uncertain aetiology that result in motor paralysis. Perhaps, the most common presentation for orthopaedic surgeons is the Parsonage-Turner syndrome typically presenting to a shoulder clinic with disordered function around the shoulder girdle and elbow and frequently scapular winging due to loss of function within the serratus anterior muscle. The history is sudden onset of severe pain following a viral prodrome, rapid loss of motor function and wasting. There is some recovery potential; but, in some cases, the paralysis is protracted with little early recovery and some permanent motor paralysis results. Nerve transfer surgery may be used to redirect motor axons from normal nerves in proximity to the paralysed muscles.
Case discussion
A 45-year-old man presented with persistent scapular winging due to poor functional recovery within the serratus anterior after an episode of viral brachial neuritis 10 months previously. Despite physiotherapy, the serratus remained non-functional. Exploration of the lower long thoracic nerve in the axilla demonstrated increased stimulation thresholds and poor recruitment. A nerve transfer using the lateral branch of the thoracodorsal nerve to latissimus dorsi coapted to the distal long thoracic nerve provided a source of motor axons. Over the next 6 months, re-innervation of the lower serratus anterior provided stability to the scapula and the winging resolved.
Tetraplegia
Trauma to the cervical spinal cord results in four-limb paralysis. The extent of paralysis in the upper limbs depends on the level of cord injury. In the mid-cervical injury, there is maybe some sparing of the C5 and C6 roots resulting in preservation of shoulder abduction and some elbow flexion. Typically, in such cases, the brachioradialis is the most distally spared muscle in the upper limb. Reconstruction of some upper limb function can be achieved through combinations of tendon transfers and tenodesis procedures.[30] Presentations are variable depending on the degree of sparing of the upper cervical roots, the dominant root innervation to key muscles and the possible partial sparing of lower roots in incomplete spinal cord injury.[31] The International Classification of the Hand in Tetraplegia (ICHT) provides a template for guiding reconstruction. The cord injury results in damage to the motor axon cell bodies within the cervical spinal cord and Wallerian degeneration results in the distal axon. Lower cervical spinal nerve roots contain cell bodies that lie below the level of the spinal cord injury, and although there is functional separation from the brain and no volitional control, the peripheral neural pathway remains intact and there is no Wallerian degeneration. The extent of the denervation depends on the length of the cervical spinal cord injury zone. Narrow injury zones with sparing of C5 and C6 allow peripheral nerve transfer from expendable fascicles or motor branches into important motor branches without denervation beyond 12 months from the injury. Prompt recognition of denervation allows transfer to motor branches emanating from the injury zone within the first 6–9 months of injury. There are theoretically additional functional gains through combining nerve transfers with tendon transfers in reconstruction of the upper limb after tetraplegic spinal cord injury. The current rehabilitation pathways may not facilitate early referral for management and there is potential for this opportunity to be lost if referral is delayed. This area is controversial because many rehabilitation specialists will defer upper limb consultation until after 18 months post-injury when they believe that the clinical situation is stabilised and motor recovery is plateauing.
In the ICHT grade 2 without triceps function, elbow extension may be achieved through transfer of the posterior deltoid to the triceps with a tendon graft and the brachioradialis tendon can be transferred for finger flexion of to the thumb for key pinch. Further, functional gains are not possible, but hand posture can be improved with a House tenodesis procedure and stabilisation of the thumb interphalangeal joint using a flexor to extensor split tendon transfer.
In the same patient, the teres minor branch or a fascicle from the axillary nerve may be transferred to the nerve to the long head of triceps for elbow extension, the brachialis nerve transferred to the AIN for active finger flexion and the supinator branches transferred to the distal PIN for active finger extension. The brachioradialis can be left as an elbow flexor and active pronator to mid-forearm rotation or rerouted as an active pronator.[32],[33]
Theoretically, greater functional status can be achieved with both active finger flexion and active extension that would be achievable with tendon transfers (active finger flexion and passive extension) without long periods of splint immobilisation necessary with tendon transfer surgery. Comparative studies are not yet available and will be needed before there is wider uptake of these reconstructive procedures.
Case discussion
A 47-year-old man sustained a burst fracture of C5 following a bicycle accident. After 12 months, he regained MRC grade 5 function in the right deltoid and unopposed flexion of the elbow with some spasticity. Brachioradialis recovered to MRC grade 4 with no distal motor function. At 14 months post-injury, he underwent surgery for transfer of a fascicle from the axillary nerve to the long head of triceps, which restored some active elbow extension by 6 months allowing him to operate a joystick control for a motorised wheelchair. Transfer of the nerve to brachialis to the median nerve restored some active finger and thumb flexion by 12 months post-surgery allowing independent feeding. Supinator branch transfer to the PIN provided tone in the digital extensors although active, volitional control was poor. The intra-operative stimulation of the PIN was poor suggesting partial lower motor neuron lesion with axonopathy due to the cervical spinal cord injury. The brachioradialis tendon was transferred to the extensor carpi radials brevis tendon resulting in active wrist extension with some tenodesis for augmentation of finger flexion and improved digital extension on passive wrist flexion.
Reconstruction After Nerve Tumour Resection
Reconstruction of the nerve gap following a tumour resection may reduce neuropathic pain; however, functional restoration is limited by a number of factors including the distance for regeneration, the length of the gap and the need for adjuvant therapies. Autologous grafts do not revascularise and support nerve regeneration if the surgical bed is irradiated. Involvement of a peripheral nerve specialist in the pre-operative planning stage may result in novel immediate reconstruction strategies using nerve transfer techniques.[34] Such options are only available when there is complete resection of some limb nerves leaving others intact.
Case discussion
A 23-year-old man presented with recurrence of an aneurysmal bone cyst of the C5 vertebral body. The C5 and C6 roots were non-functional from compression by tumour. The vertebral column was reconstructed with a cage and bone grafting. Peripheral nerve reconstruction was performed with a medial spinal accessory branch transfer to the suprascapular nerve through a posterior approach, medial triceps branch transfer to the axillary nerve and double fascicle transfer from the median and ulnar nerves to the nerve to biceps and nerve to brachialis, respectively.
Conclusions
Nerve transfer is an accepted technique for motor reconstruction following severe peripheral nerve injury. Nerve transfer may be used successfully in other causes of paralysis although the criteria for success still need to be determined in cases of nerve entrapment. The window of opportunity for successful reconstruction may be longer than for a complete traumatic nerve lesion due to preservation of some motor function within the residual nerve and adaptive change at the neuromuscular junction. Quantitative electromyography to determine the size of residual motor units may provide important information regarding the potential responsiveness of a non-functional muscle to re-innervation and should form the basis of further study. Clinical comparative studies need to be undertaken to assess the possibility of functional gains from improved motor strength through re-innervation of non-functional (MRC 1–3) grades following compressive motor radiculopathy that has failed to improve with time and physiotherapy.
Ethical considerations
Ethical considerations were complete in conjunction with the Hospital Ethical Board.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Author's contribution
DP, MN, DC, PJ and PM are contributed to the literature search, Each author went through case notes to find a suitable case for each example. The structuring of the article and overall writing of the piece including proof reading and editing involved all authors. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
| 1. | Tuttle HK. Exposure of the brachial plexus with nerve transplantation. JAMA 1913;61:15-7. [Google Scholar] |
| 2. | Vulpius O, Stoffel A. Operationen am nervensystem. Orthopadische Operationslhere. Stuttgart: Publshed by Ferdinand Enke; 1913. [Google Scholar] |
| 3. | Holmes W, Young JZ. Nerve regeneration after immediate and delayed suture. J Anat 1942;77:63-96.1. [Google Scholar] |
| 4. | Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged denervation. J Neurosci 1995;15:3886-95. [Google Scholar] |
| 5. | Fu SY, Gordon T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged axotomy. J Neurosci 1995;15:3876-85. [Google Scholar] |
| 6. | Swanson AN, Wolfe SW, Khazzam M, Feinberg J, Ehteshami J, Doty S, et al. Comparison of neurotization versus nerve repair in an animal model of chronically denervated muscle. J Hand Surg Am 2008;33:1093-9. [Google Scholar] |
| 7. | Gordon T, de Zepetnek JET. Motor unit and muscle fiber type grouping after peripheral nerve injury in the rat. Exp Neurol 2016;285:24-40. [Google Scholar] |
| 8. | Brunelli G, Brunelli F. Partial selective denervation in spastic palsies (hyponeurotization). Microsurgery 1983;4:221-4. [Google Scholar] |
| 9. | Witoonchart K, Leechavengvongs S, Uerpairojkit C, Thuvasethakul P, Wongnopsuwan V. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, part I: An anatomic feasibility study. J Hand Surg Am 2003;28:628-32. [Google Scholar] |
| 10. | Colbert SH, Mackinnon S. Posterior approach for double nerve transfer for restoration of shoulder function in upper brachial plexus palsy. Hand (N Y) 2006;1:71-7. [Google Scholar] |
| 11. | Bertelli JA, Kechele PR, Santos MA, Duarte H, Ghizoni MF. Axillary nerve repair by triceps motor branch transfer through an axillary access: Anatomical basis and clinical results. J Neurosurg 2007;107:370-7. [Google Scholar] |
| 12. | Bertelli JA, Ghizoni MF. Nerve transfer from triceps medial head and anconeus to deltoid for axillary nerve palsy. J Hand Surg Am 2014;39:940-7. [Google Scholar] |
| 13. | Oberlin C, Béal D, Leechavengvongs S, Salon A, Dauge MC, Sarcy JJ, et al. Nerve transfer to biceps muscle using a part of ulnar nerve for C5-C6 avulsion of the brachial plexus: Anatomical study and report of four cases. J Hand Surg Am 1994;19:232-7. [Google Scholar] |
| 14. | Loy S, Bhatia A, Asfazadourian H, Oberlin C. Ulnar nerve fascicle transfer onto to the biceps muscle nerve in C5-C6 or C5-C6-C7 avulsions of the brachial plexus. Eighteen cases. Ann Chir Main Memb Super 1997;16:275-84. [Google Scholar] |
| 15. | Oberlin C, Ameur NE, Teboul F, Beaulieu JY, Vacher C. Restoration of elbow flexion in brachial plexus injury by transfer of ulnar nerve fascicles to the nerve to the biceps muscle. Tech Hand Up Extrem Surg 2002;6:86-90. [Google Scholar] |
| 16. | Liverneaux PA, Diaz LC, Beaulieu JY, Durand S, Oberlin C. Preliminary results of double nerve transfer to restore elbow flexion in upper type brachial plexus palsies. Plast Reconstr Surg 2006;117:915-9. [Google Scholar] |
| 17. | Mackinnon SE, Novak CB, Myckatyn TM, Tung TH. Results of reinnervation of the biceps and brachialis muscles with a double fascicular transfer for elbow flexion. J Hand Surg Am 2005;30:978-85. [Google Scholar] |
| 18. | Ray WZ, Pet MA, Yee A, Mackinnon SE. Double fascicular nerve transfer to the biceps and brachialis muscles after brachial plexus injury: Clinical outcomes in a series of 29 cases. J Neurosurg 2011;114:1520-8. [Google Scholar] |
| 19. | Dy CJ, Garg R, Lee SK, Tow P, Mancuso CA, Wolfe SW, et al. Asystematic review of outcomes reporting for brachial plexus reconstruction. J Hand Surg Am 2015;40:308-13. [Google Scholar] |
| 20. | Merrell GA, Barrie KA, Katz DL, Wolfe SW. Results of nerve transfer techniques for restoration of shoulder and elbow function in the context of a meta-analysis of the English literature. J Hand Surg Am 2001;26:303-14. [Google Scholar] |
| 21. | Zhang L, Dong Z, Zhang CL, Gu YD. Surgical anatomy of the radial nerve at the elbow and in the forearm: Anatomical basis for intraplexus nerve transfer to reconstruct thumb and finger extension in C7 - T1 brachial plexus palsy. J Reconstr Microsurg 2016;32:670-4. [Google Scholar] |
| 22. | Xu B, Dong Z, Zhang CG, Gu YD. Multiple nerve and tendon transfers: A new strategy for restoring hand function in a patient with C7-T1 brachial plexus avulsions. J Neurosurg 2017;127:837-42. [Google Scholar] |
| 23. | Li Z, Reynolds M, Satteson E, Nazir O, Petit J, Smith BP, et al. Double distal intraneural fascicular nerve transfers for lower brachial plexus injuries. J Hand Surg Am 2016;41:e15-9. [Google Scholar] |
| 24. | Brown JM, Tung TH, Mackinnon SE. Median to radial nerve transfer to restore wrist and finger extension: Technical nuances. Neurosurgery 2010;66:75-83. [Google Scholar] |
| 25. | Ray WZ, Mackinnon SE. Clinical outcomes following median to radial nerve transfers. J Hand Surg Am 2011;36:201-8. [Google Scholar] |
| 26. | Lee EY, Karjalainen TV, Sebastin SJ, Lim AY. The value of the tender muscle sign in detecting motor recovery after peripheral nerve reconstruction. J Hand Surg Am 2015;40:433-7. [Google Scholar] |
| 27. | Wang Y, Zhu S. Transfer of a branch of the anterior interosseus nerve to the motor branch of the median nerve and ulnar nerve. Chin Med J (Engl) 1997;110:216-9. [Google Scholar] |
| 28. | Novak CB, Mackinnon SE. Distal anterior interosseous nerve transfer to the deep motor branch of the ulnar nerve for reconstruction of high ulnar nerve injuries. J Reconstr Microsurg 2002;18:459-64. [Google Scholar] |
| 29. | Barbour J, Yee A, Kahn LC, Mackinnon SE. Supercharged end-to-side anterior interosseous to ulnar motor nerve transfer for intrinsic musculature reinnervation. J Hand Surg Am 2012;37:2150-9. [Google Scholar] |
| 30. | Fox IK, Davidge KM, Novak CB, Hoben G, Kahn LC, Juknis N, et al. Use of peripheral nerve transfers in tetraplegia: Evaluation of feasibility and morbidity. Hand (N Y) 2015;10:60-7. [Google Scholar] |
| 31. | Brown JM. Nerve transfers in tetraplegia I: Background and technique. Surg Neurol Int 2011;2:121. [Google Scholar] |
| 32. | Mackinnon SE, Yee A, Ray WZ. Nerve transfers for the restoration of hand function after spinal cord injury. J Neurosurg 2012;117:176-85. [Google Scholar] |
| 33. | Fox IK, Davidge KM, Novak CB, Hoben G, Kahn LC, Juknis N, et al. Nerve transfers to restore upper extremity function in cervical spinal cord injury: Update and preliminary outcomes. Plast Reconstr Surg 2015;136:780-92. [Google Scholar] |
| 34. | Brown JM, Mahan MA, Mandeville R, Carter BS. Establishing reconstructive neurosurgery as a subspecialty. Neurosurg Focus 2017;43:E7. [Google Scholar] |
Fulltext Views
1,993
PDF downloads
480





